Reduce Research Time and Frustration
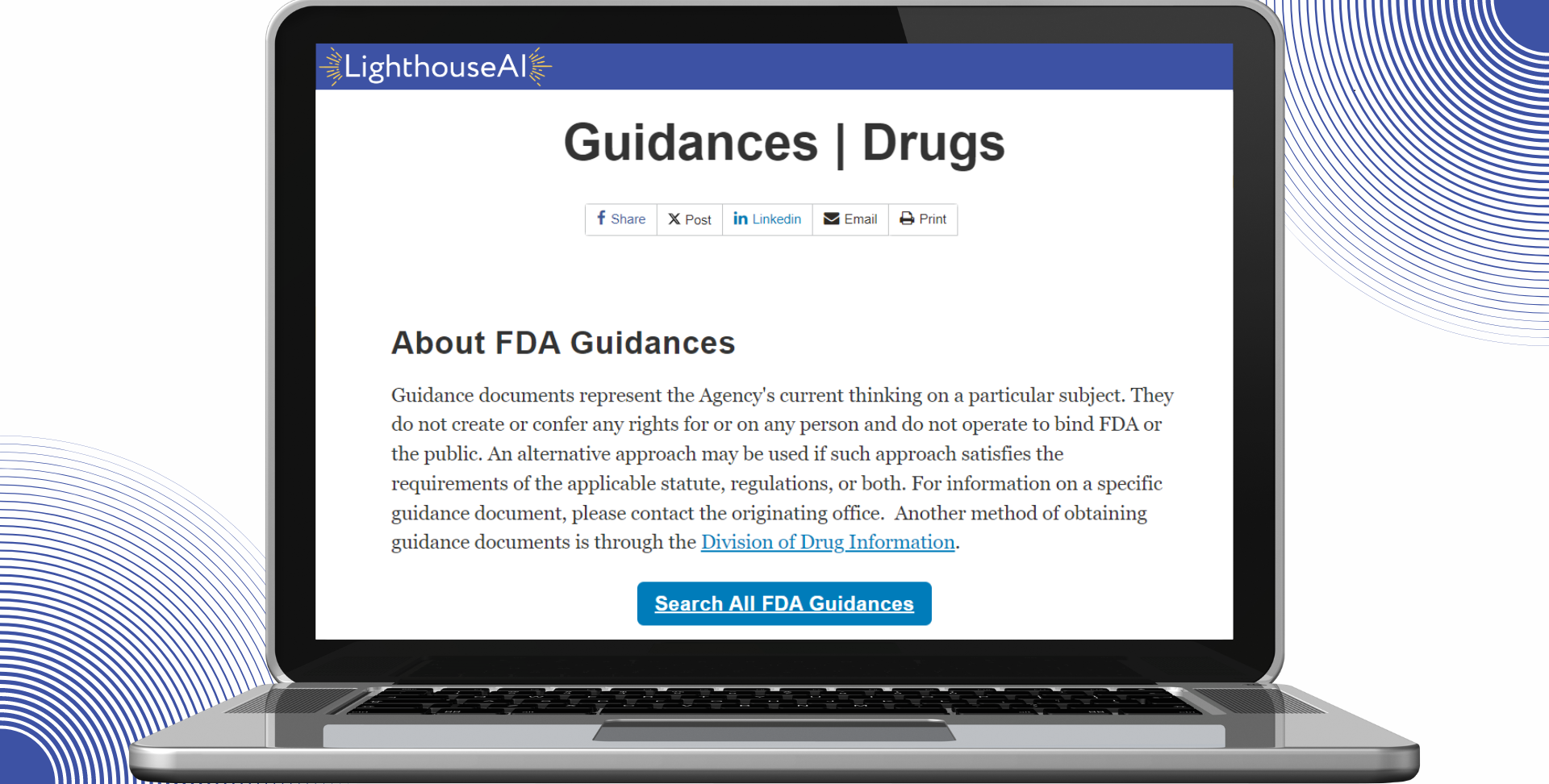
Cut down the time spent researching regulatory requirements. LighthouseAI Citations links you directly to the requirement details issued by state and federal agencies, taking you directly to the source.
Reduce wasted time and frustration scouring regulatory agency websites for the details of specific regulatory requirements with LighthouseAI Citations.
Improved Understanding of Your Requirements
Gain added depth and clarification into your state and federal requirements. LighthouseAI’s Citations feature links you directly to the official documentation issued by regulatory agencies.
With easy access to official documentation, you spend less time researching, and more time becoming knowledgeable about your specific requirements.
Let’s Talk Compliance
Click the button below to schedule a Discovery Call with our Compliance Experts
Reduce Your Compliance Stress and Workload
Automate Your Compliance Research.
Run Instant Compliance Assessments.
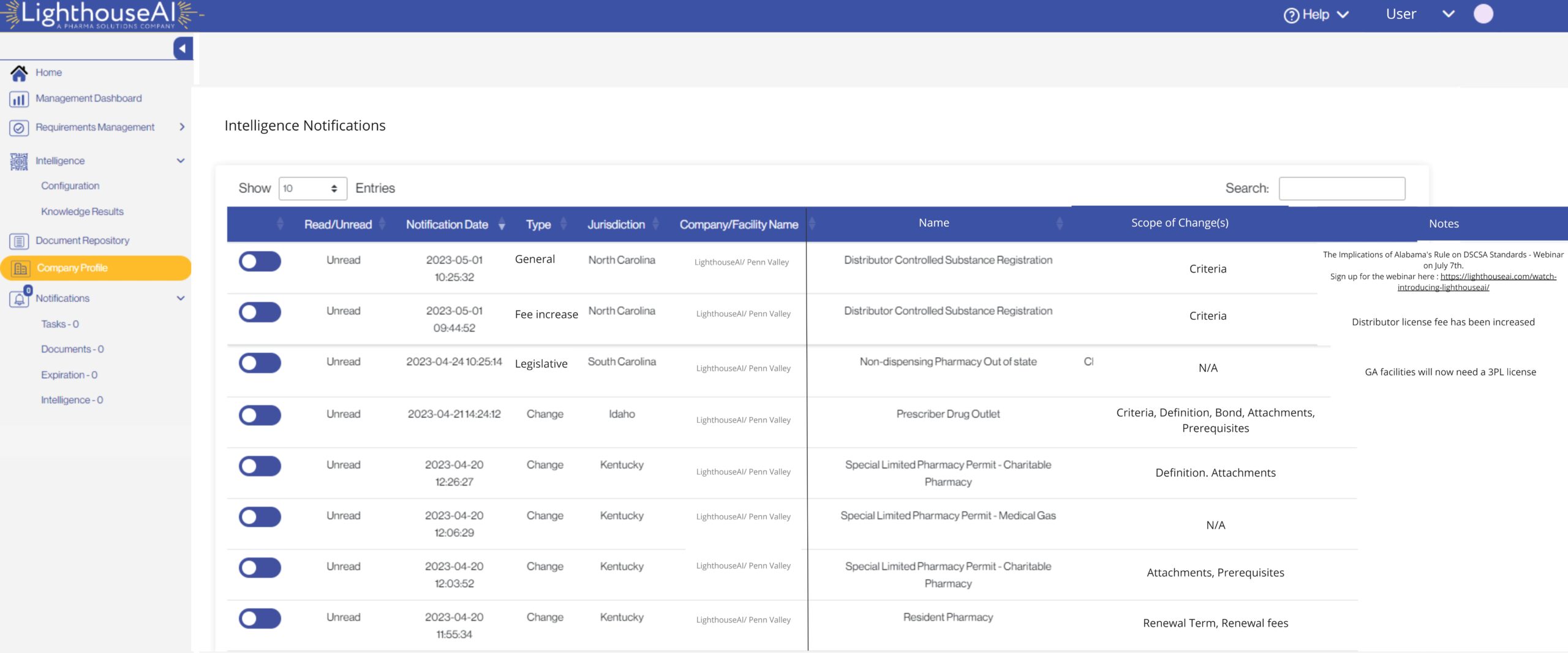
Receive Automated Regulatory Notifications.
Centralize Compliance Data to One Platform.
Track & Manage All Compliance Requirements.
Store Important & Sensitive Documents Securely.