Pharmaceutical State Licensing Software and Services for the Pharma Supply Chain
LighthouseAI combines advanced state licensing software with expert services to centralize, simplify, and ensure accuracy across every state and jurisdiction.
Get actionable notices for renewals, filings, and rule changes. Assign owners and due dates.







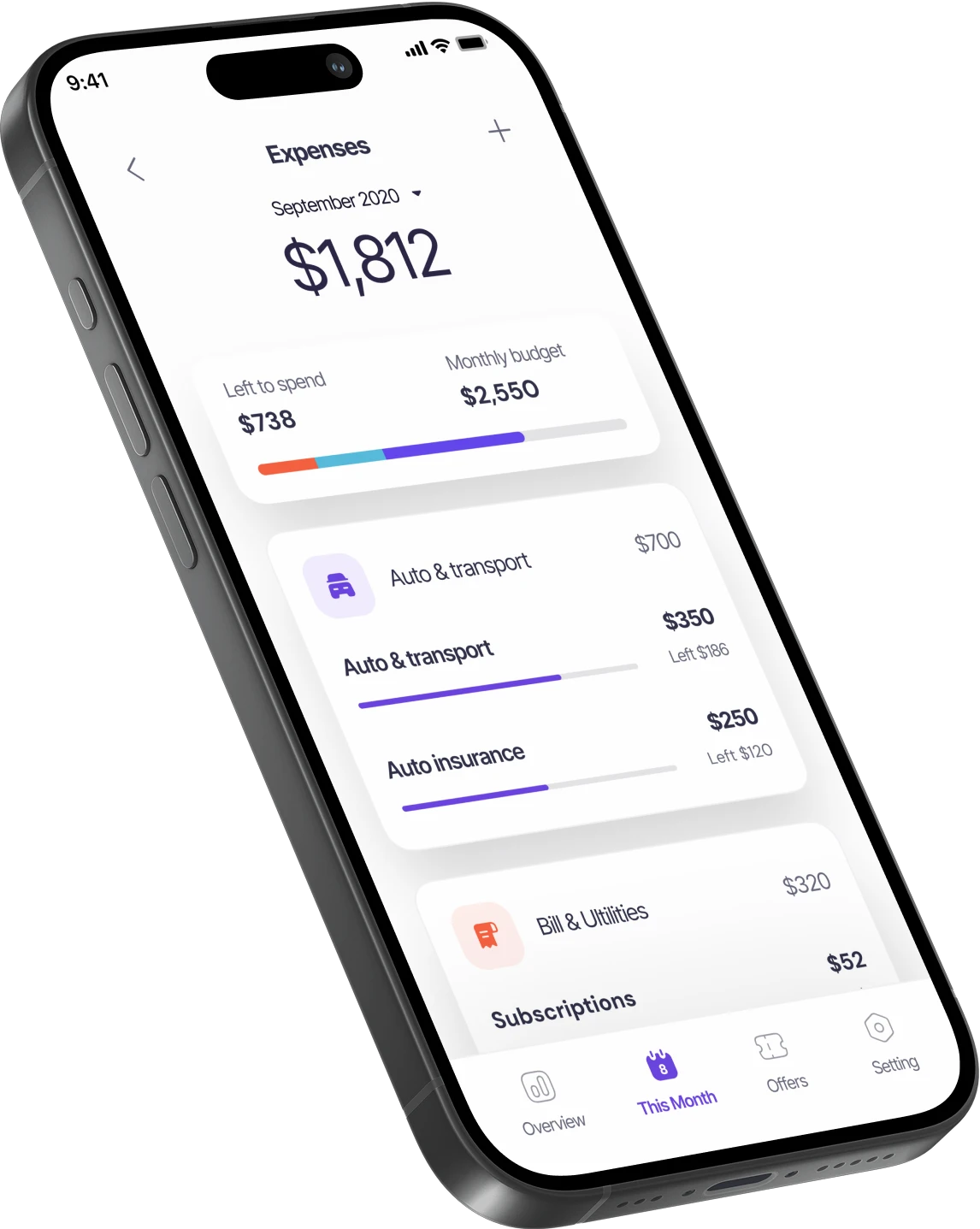
Lorem ipsum dolor sit amet consectetur molestie ullamcorp elit non diam at pharetra integer non fringilla. Non cras sapien rutrum maecenas tellus posuere faucibus tincidunt.
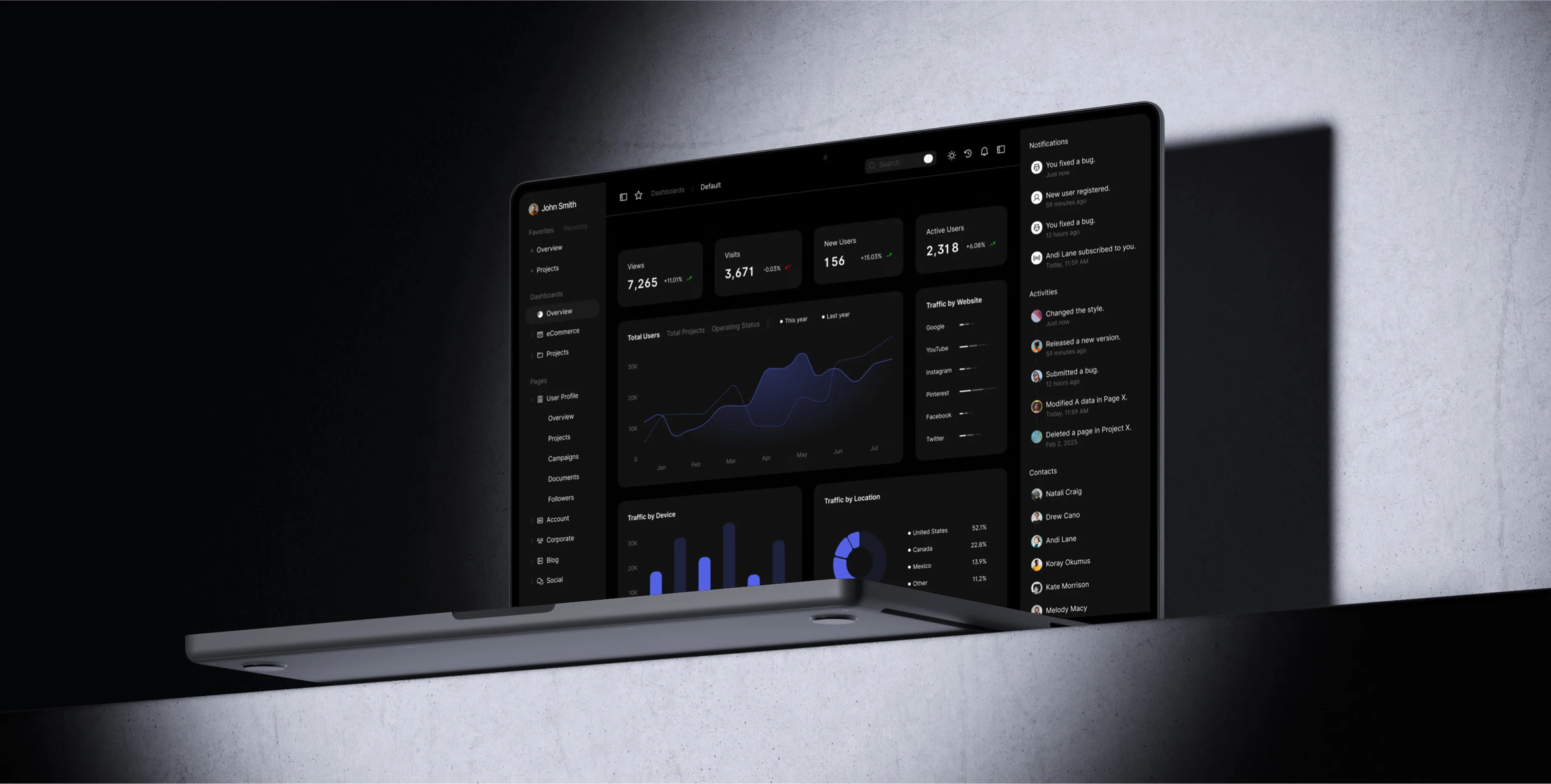
Navigate every state’s licensing maze with seasoned experts who prepare filings, manage fingerprints, resolve agency questions, and keep approvals on track.
Uncover exactly where you stand. Our team benchmarks compliance, researches statutes, and delivers actionable reports with clear remediation plans.
Achieve NABP accreditation with confidence. We build compliant policies, train staff, perform readiness audits, and guide you through successful review.
From URAC to ACHC, we streamline pharmacy accreditations by aligning operations, preparing documentation, training teams, and supporting surveys.
Expand into Puerto Rico without the red tape. We prepare bilingual filings, coordinate agencies, manage renewals, and monitor inspection readiness.
Control substance compliance done right. Experts create SOPs, conduct audits, train staff, and resolve DEA gaps before they become violations.
Avoid surprises on inspection day. We perform mock reviews, assemble evidence, coach staff, and provide corrective action plans that satisfy regulators.
.webp)